Get updates delivered to you daily. Free and customizable.
AccuWeather
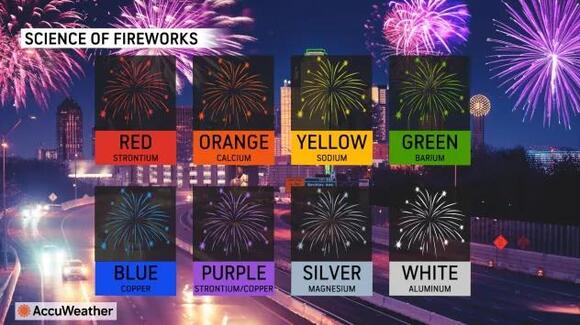
What makes fireworks burst with vibrant colors?
By Brian Lada,
10 days agoExpand All
Read in NewsBreak
Comments / 0
Add a Comment
YOU MAY ALSO LIKE
 Most Popular
Most PopularVirginia State8 hours ago
Total Apex Sports & Entertainment13 days ago
Total Apex Sports & Entertainment8 days ago
Total Apex Sports & Entertainment10 days ago
AccuWeather2 days ago
AccuWeather1 day ago
Total Apex Sports & Entertainment1 day ago
Bucks County Beacon4 days ago
Wyoming State29 days ago
Get updates delivered to you daily. Free and customizable.
Welcome to NewsBreak, an open platform where diverse perspectives converge. Most of our content comes from established publications and journalists, as well as from our extensive network of tens of thousands of creators who contribute to our platform. We empower individuals to share insightful viewpoints through short posts and comments. It’s essential to note our commitment to transparency: our Terms of Use acknowledge that our services may not always be error-free, and our Community Standards emphasize our discretion in enforcing policies. We strive to foster a dynamic environment for free expression and robust discourse through safety guardrails of human and AI moderation. Join us in shaping the news narrative together.


Comments / 0